
Maize Lethal Necrosis: Building a comprehensive response
In September 2011, reports came of a devastating new maize disease in the Southern Rift Valley of Kenya. The symptoms were described as mottling of the leaves, small cobs with few grains, and necrosis of young leaves leading to “dead heart” and eventually plant death. It was common for affected fields to show 100 percent infection rates, meaning that some farmers faced losing the entire crop.
 By October 2012, a study team sent by CIMMYT and the Kenya Agricultural and Livestock Research Organization (KALRO) confirmed the disease to be maize lethal necrosis (MLN).
By October 2012, a study team sent by CIMMYT and the Kenya Agricultural and Livestock Research Organization (KALRO) confirmed the disease to be maize lethal necrosis (MLN).
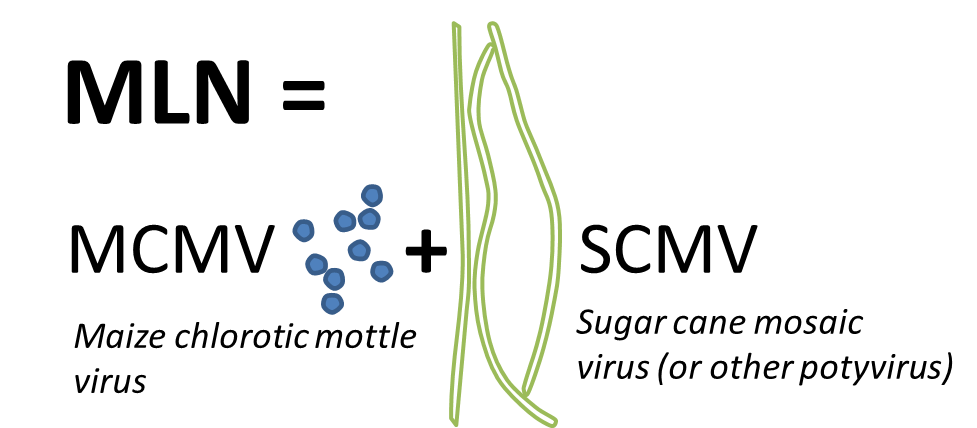
MLN is a disease caused by the synergistic combination of Maize Chlorotic Mottle Virus (MCMV) and any from the potyvirus family, in this case Sugarcane Mosaic Virus (SCMV). SCMV is found worldwide, but this was the first report of either MCMV or maize lethal necrosis in Africa.
By then, for reasons that remain unclear, the disease was spreading quickly to other districts of Kenya, and at higher altitudes than thought possible. Between 2012 and 2014, it was found in Tanzania, Uganda, South Sudan, the Democratic Republic of Congo and then Ethiopia. The USDA reports that at this rate, the disease will spread and intensify across Africa by 2020.
An upcoming publication from the CIMMYT Socioeconomics team indicates that 23 percent of Kenya’s maize production was lost to MLN in 2014, about 2.1 million metric tons. How can the tide of this disease be turned in Africa, and similar devastation prevented elsewhere?
In search of resistance
In the Biosafety Laboratory at CIMMYT headquarters, El Batán, Monica Mezzalama cuts up some MLN-infected maize leaves and crushes them into a buffer solution. Passing through two further sets of safety doors, she arrives at the greenhouse where several hundred young maize plants await inoculation. The plants are dusted with a fine grey powder to cause surface damage, allowing the disease-causing viruses to enter when Mezzalama brushes the youngest leaf of each plant with the solution of infected plant matter.
This is the beginning of a systematic process to find sources of MLN resistance from samples in the Genebank at CIMMYT.
As Terry Molnar, maize breeder at CIMMYT explains: “The maize industry has always been very fast at responding to diseases, but this has been difficult in the case of MLN. What’s scaring people is that it’s spreading so fast in Eastern Africa, the two viruses that cause it are common across all tropical maize-growing countries, and we’re not finding many sources of resistance.”
Luckily, the geographic information attached to maize accessions within the CIMMYT collection provides a map to finding resistance; the one thousand accessions being tested are landraces chosen from areas known to be affected by MCMV in Latin America and the Caribbean. After the first round of testing, several of these accessions remained healthy after inoculation, and will be tested further to confirm their resistance. However, depending on the level of efforts that can be funded, it may take several years to turn resistant maize landraces into elite breeding material.
Reacting on all fronts
The rapid emergence of MLN in East Africa was met by an equally impressive effort to quickly offer farmers varieties capable of surviving MLN by identifying sources of tolerance further down the breeding timeline.
With funding from the Bill and Melinda Gates Foundation (BMGF), the Syngenta Foundation for Sustainable Agriculture (SFSA) and MAIZE CRP, CIMMYT and KALRO jointly established an MLN screening facility at Naivasha, Kenya in September 2013. This facility now widely used by the public and private sector.
After a mass screening of 21,000 pre-commercial hybrids, 26,000 inbred maize lines and 80 commercial cultivars, five hybrids with moderate tolerance to MLN are already in varying stages of commercial release in Kenya, Uganda and Tanzania.
“This first generation of hybrids is based on chance finds in available germplasm. The second generation will come from the inbred lines that we are developing,” said B.M. Prasanna, director of the CIMMYT Global Maize Program and now also MAIZE CRP. “We are also incorporating resistance into as many as 25 commercial lines using genetic marker-assisted backcrossing.”
New options on the maize seed market offer an immediate opportunity for farmers to control the impact of MLN in their fields, but a long-term strategy is still needed to secure the crop’s future.
“This was good as an emergency response, because we needed to react. But in the long run we need to develop diverse sources of material with greater resistance to MLN,” said Mezzalama. The targeted search for sources of MCMV resistance in the genebank aims to ensure a diverse supply of fully MLN-resistant hybrids for the future.
Beyond seed
In the meantime, it is essential to complement the response from plant breeders with a greater understanding of how the disease emerged, and therefore how it can be controlled. At a conference held in Nairobi from 12-14 May 2015, it became clear just how quickly this understanding is evolving.
 Earlier, studies had found that seed transmission did not play a role in the spread of MCMV in the US, but the experience in Kenya suggests otherwise. “The fact that it is transmitted by seed makes control more difficult. This poses a big challenge to seed processors, and phytosanitary services in the region,” said Mezzalama. In May, CIMMYT produced a set of recommendations on ‘MLN Pathogen Diagnosis, MLN-free Seed Production and Safe Exchange to Non-Endemic Countries.’
Earlier, studies had found that seed transmission did not play a role in the spread of MCMV in the US, but the experience in Kenya suggests otherwise. “The fact that it is transmitted by seed makes control more difficult. This poses a big challenge to seed processors, and phytosanitary services in the region,” said Mezzalama. In May, CIMMYT produced a set of recommendations on ‘MLN Pathogen Diagnosis, MLN-free Seed Production and Safe Exchange to Non-Endemic Countries.’
Breaking the disease transmission cycle in the field is also important. In the affected area of Kenya, maize is grown continuously throughout the year, meaning that insect vectors can carry the viruses from crop to crop, season to season. “Many weeds are also susceptible to MCMV, and even sugarcane and millet can host the virus. Planting non-host crops will help reduce the inoculum reservoir,” said Mezzalama.
MAIZE CRP is funding a project with the International Centre of Insect Physiology and Ecology (ICIPE) to further investigate insect vector dynamics through its competitive grants initiative (CGI). CGI funds have also been granted to the Federal University of Nigeria to help prevent the spread of MLN elsewhere in Africa, with support from the International Institute for Tropical Agriculture (IITA). Plans are afoot to set up an MLN quarantine facility at Harare, Zimbabwe.
MLN poses an existential threat to maize food systems but, with the right resources, plant pathologists and breeders are well-equipped to face such challenges. “We often cannot predict disease outbreaks,” said Mezzalama, “but we have to react quickly when such crises arise, as was also recently the case of wheat stem rust.”
The international partnership to develop a comprehensive response to MLN continues to make progress on all fronts. The next phase will build capacities in sub-Saharan Africa by creating a transnational ‘community of practice’ among phytosanitary agencies and giving the commercial seed sector, especially small-to-medium size companies, access to MLN diagnosis and the ability to produce clean seed for farmers.






